[vc_row][vc_column][vc_custom_heading text=\”Intergranular Corrosion\” use_theme_fonts=\”yes\”][vc_custom_heading text=\”Background\” font_container=\”tag:h3|text_align:left\” use_theme_fonts=\”yes\”][vc_column_text]
Austenitic Stainless Steels present corrosion resistance ability due to high chromium (Cr) content. Chromium naturally forms a thin, adherent and very protective passive oxide layer when exposed into corrosive aqueous environment which is primarily the reason of its high resistance capabilities.
When austenitic stainless is exposed to a corrosive aqueous environment, chromium oxide enriches at the metal interface due to the formation of passive and resistent corrosion layer.
[/vc_column_text][vc_custom_heading text=\”Problem\” font_container=\”tag:h3|text_align:left\” use_theme_fonts=\”yes\”][vc_column_text]
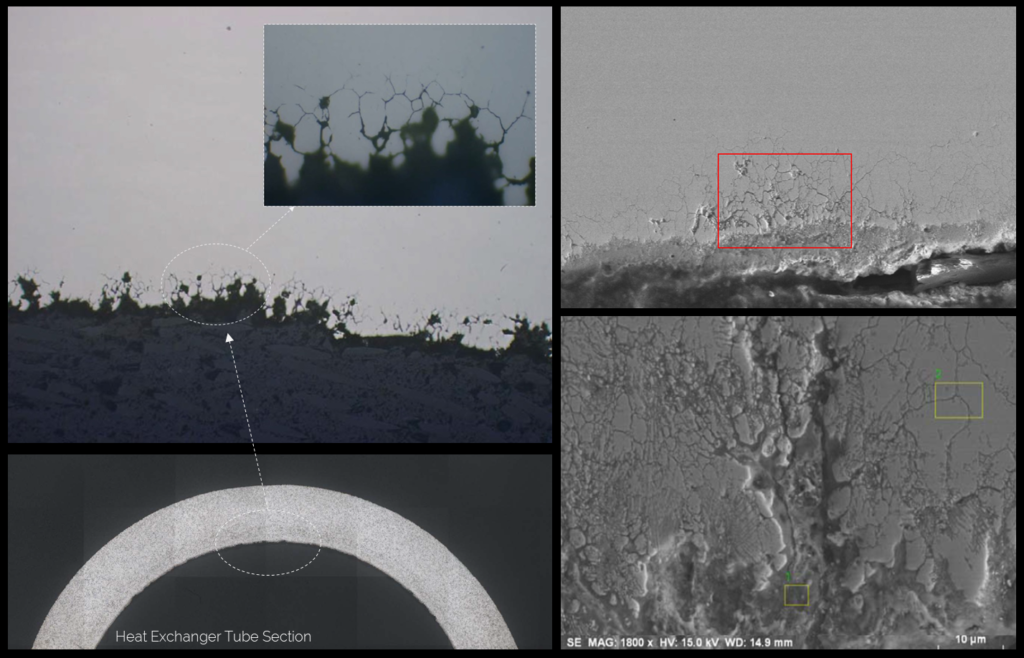
However, if the material is heated into a range of 510-790ºC, chromium combines with carbon to form Cr-Carbide (Cr23C6) which may precipitates at the grain boundaries and create a general disorder in the main structural geometry of the base material.
As a result, the steel might present microfissures and intergranular channels of material detachments which will make the tube to be prone to suffer several forms of corrosion.
Therefore the material is said to be “sensitized”. Sensitization is attributed due the alloy element composition, degree of temperature the material reaches and the time of heat exposure.
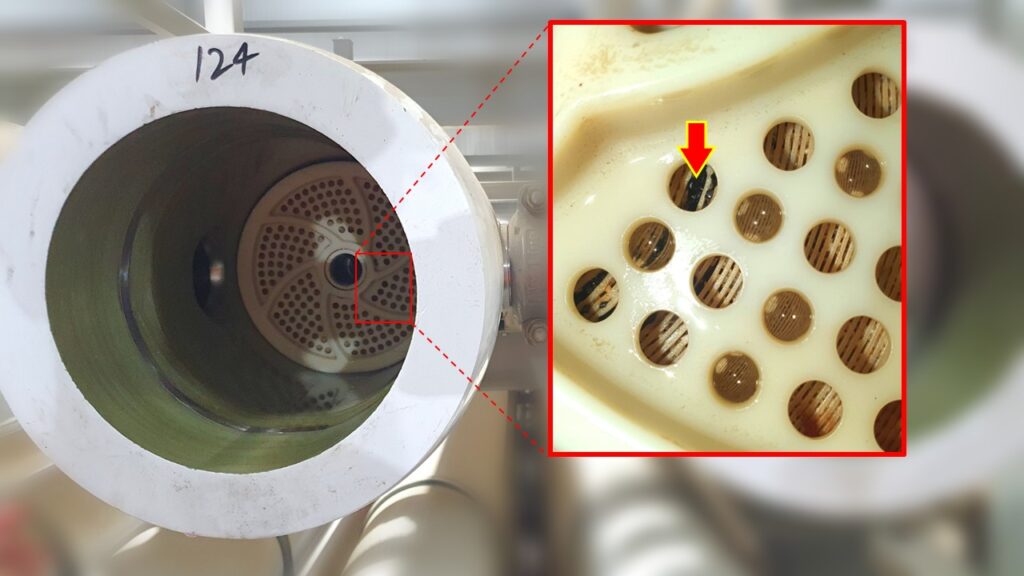
In the photo it is shown a case of intergranular selective attack where the corrosion phenomenon advances toward the inside of the tube and progressing along the grain of the metal.
As a result, the grain loses its alloying capabilities and becomes anode with respect surrounding grains.
[/vc_column_text][vc_single_image image=\”11921\” img_size=\”full\”][/vc_column][/vc_row]