A sudden change in a fluid cross-section area provokes a dynamic condition in which water pressure drops sharply below its boiling point and produces instant vaporization.
Since this process is adiabatic, there is no exchange of heat with its surroundings, therefore, the total enthalpy remains the same. Consequently, most of the water molecules will absorb the bulk of that energy as latent heat and will make water turn into steam.
This phenomenon brings about many destructive effects related to chemical corrosion attack, which is one of the most common issues found in steam water systems. Subsequently, it can derive to accumulation of sediment in the cycle, under-deposit corrosion, overheating, caustic corrosion, control valve obstructions or spray nozzle cloggins to mention a few cases.
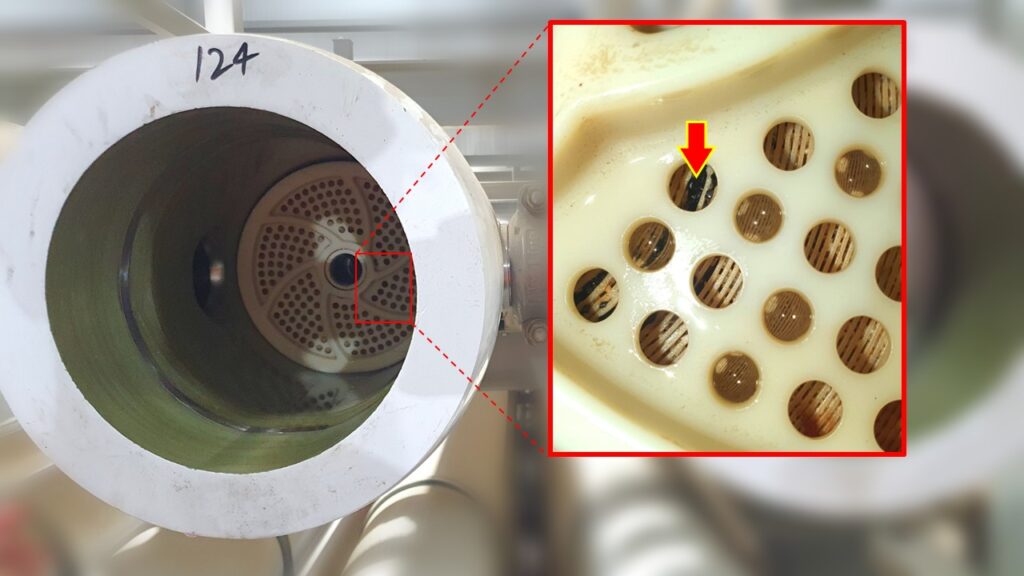
The problem is likely encountered in LP drain motorized valves feeding flash tanks, spray nozzles and diffusers at bypass attemperations, orifice plates, nozzle or pitot flowmeters, LP flash tank drains to condenser, blowdown systems. We have also observed the same type of corrosion patterns at the deaerator nozzles. Image 1 represents the cross section top view of a HRSG preheater module It also shows how temperatures vary at the back-end tubes. The increase of velocity in the flue gas would produce gradients of pressure between the two opposite sides of the tube. In consequence, this would eventually create a vortex effect and turbulences in the backside of the tubes. This is shown in image 2.
Turbulence would drop velocity down to zero near the surface, which would decrease the rate of energy transfer from the flue gas to the condensate. By the same token, the lack of flue gas circulation around the tubes would bring temperatures down below the dew point and end up creating droplets of condensation. Image 3 shows areas of high probability for this phenomenon to occur. In that event, a layer of condensation would develop all along the surface of the tubes, while drastically altering the heat convection and thermal conduction rate across the wall. It is empirically proven that solubility of chemical species is actively dependent on pressure and temperature, among other properties. Although there is not a direct correlation to predict such dependency, it has been observed that, for most compounds, solubility falls with a decrease in those properties.
As water expands into a pressure below the boiling point, some impurities will exceed its limit of saturation and precipitate. In fact, these precipitates are deposits of salt compounds which can be very aggressive to the equipment. Depending on the nature of the accumulation, the affected zone can develop several types of corrosion patterns. Hence, sodium salts can undergo hydrolysis and produce caustic corrosion. Silica precipitates to form a very hard and impervious scale which can derivates into stress corrosion cracking. Sodium carbonate or sodium chloride can be adsorbed on the surface of the metal affecting the passivation protective layer. Under-deposit corrosion would be expected to happen in most of cases as a result of electrochemical reactions underneath deposition of salts.
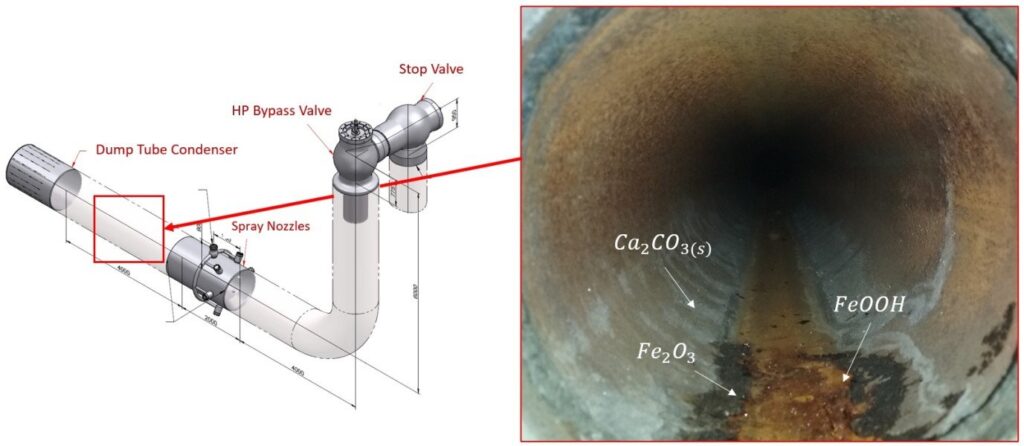
I took the attached photo during a inspection to show calcium carbonate deposits (light white patterns) encountered downstream the HP spray attemperation bypass valve.
Air in-leakage in the condenser, caused high level of carbon dioxide dissolved in water, in the form of carbonic acid ions. Subsequently, the carbonic acid ions combined with impurities of calcium dissociated in water to form calcium carbonate. In the even of an abrupt expansion, which is the case of the spray attemperation, the equilibrium among the chemical species dissolved in water is broken. Some ions will vaporize and escape as carryovers into the steam flow, while other impurities will exceed its solubility under the given conditions and precipitate as insoluble salts.
Refer to the following post to find extra information about this type of failure, click here